WE'VE MOVED!
CHECK OUT OUR NEW WEBSITE BELOW
SECOND SOURCE MEDICAL LLC
As an FDA-registered contract manufacturer for Class II 510(k) and Class III PMA products, Second Source Medical provides decades of engineering and quality excellence to support your success in product development, regulatory approval, rapid time-to-market, and mass production.
We specialize in contracted design and production services for complex mechanical/electromechanical and combination devices, including angioplasty equipment and catheters, for your applications such as neurovascular, cardiovascular, urological and endoscopic intervention or surgery.
As your trusted partner, our services encompass rapid response of component design/fabrication to finished devices, process development and validations, including special controlled environments for pilot and mass production. We also provide lab space, office space and CER to make our facility your HQ.
At Second Source, we’re committed to supporting your success. We are proud to provide our clients with the best possible service and continue to build long term relationships.

DESIGN
Complex and high-performance components for microcatheters, balloon catheters; braided or coiled wire; functional prototypes
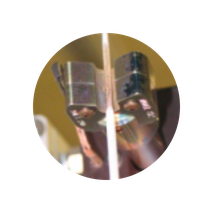
MANUFACTURE
Process development, optimization, validations and scale-up production from first-article to thousands of units

MANAGE
Project and quality management from napkin to first-in-man, regulatory submissions support, high-volume commercialization
MANAGEMENT MESSAGES
We wish good health to everyone during this COVID pandemic. It has been a very challenging two years for us and our clients, as many were affected by the slowdown of medical procedure volume, clinical studies enrollment, supply chain disruption, and labor shortages or other difficulties.
Despite all these challenges, Second Source Medical continues to thrive and see new opportunities with the arrival of several new client startups. We are focusing on mitigation of supply chain challenges and pushing for progress on customers’ projects. We continue to offer manufacturing and operations horsepower while also providing development, quality and sourcing support.
Kicking off a new medical device company requires partnership and trust. We know not all programs start with a 7-figure budget. At SSM, some of our biggest projects started with an idea and a little sweat. We aim at being a synergistic force to help our customers’ teams achieve their short & long-term goals.
EXCITING NEWS!
Second Source Medical is very pleased to announce that we have become a Bay Area family of companies that have joined hands to provide premium design, development and production capabilities – now offering complete one-stop service for advanced balloon and catheter technologies for startup and large clients to take their projects from napkin to prototype and commercialization.
MediBalloon (www.mediballoon.com) designs and produces custom extrusions and balloons for medical applications, including cardiovascular, peripheral vascular, neurovascular procedures, ear nose and throat (ENT) and gastro-intestinal (GI) operations.
MedeonBio (www.MDNBio.com) provides services for complex catheter design and assembly, utilizing state of the art processes, such as braiding, coil winding, vertical laminating, hot die bonding, laser welding, balloon bonding, pleating, tipping and swaging for cardiovascular, neurovascular, structural hearts, urology and cardiac surgery applications.
COVID SAFETY ISSUES
We are committed to providing a safe and productive work environment for our employees and staff members of our on-site clients. We follow both CDC&P and County of Santa Clara guidelines regarding building access and mask/distancing requirements. We certainly can work out a safety protocol to fit the needs of our clients.
OPEN POSITIONS
SSM has a few new openings throughout the company. Currently we are looking for a QA engineer, project manager, manufacturing associates and manufacturing engineers. Please feel free to submit your resume to the SSM Human Resources department at: hrbp@secondsourcemed.com
